I invite you to explore my portfolio, which offers a glimpse into some of my published works. Each piece of content is meticulously crafted to be scientifically accurate and adheres to the latest research guidelines. It is designed to provide clear and concise information, empowering the target audience to make informed decisions.
- Health Blog
- Health Content
- Case Report and Summaries
- Plain Language Summaries
- Manuscript Writing
- Clinical Study Protocol
- Clinical Trial Report
- Informed Consent Form
- Investigator Brochure
- Package Insert
- Infographics
- Continuing Medical Education
- Scientific Poster
- Health Journalism Article
- Health Journalism Interview
Health Blog
This SEO-compliant blog is written for a client whose target audience is the American population. The aim was to increase the outreach and help the client’s website stand high on Google rankings.
Health Content
This article is crafted clearly and concisely for a general audience. It aims to educate and establish trust so that they can take informed decisions for their health.
Case Report and Summaries
This case study is designed alongside the publication guidelines. This is meant for healthcare professionals to be enlightened about the novel therapy that looks promising in the digital healthcare world.
Plain Language Summaries
The goal of this document is to help readers, like patients, caregivers, or the general public, understand medical information and make smart choices, without getting confused by complicated details.
Manuscript Writing
This clinical trial manuscript aims to communicate trustworthy, evidence-based findings of the study that can improve understanding of healthcare practices, guide treatment decisions, and inspire further research. It is aimed at healthcare professionals, researchers, and policymakers.
Clinical Study Protocol
This submission-ready clinical study plan is written abiding to the regulatory guidelines. It provides a detailed framework for conducting a clinical trial and helps ensure the study is scientifically valid and ethically sound.
Clinical Study Protocol (Sample)
Clinical Trial Report
This submission-ready clinical trial report is written to communicate findings accurately and comprehensively, adhering to the regulatory guidelines, and supporting scientific understanding, patient care, regulatory processes, and future research.
Informed Consent Form
This informed Consent form is written for participants of a clinical trial to understand the study’s nature, risks, benefits, and rights before they agree to take part in the study. The language is simple and complies with regulatory and ethical standards.
Patient Information Sheet And Informed Consent Form (Sample)
Investigator Brochure
This investigator brochure is written to support the clinical trial process and guide investigators, clinical trial staff, and regulatory bodies in the safe and effective conduct of the trial.
Package Insert
This package insert is written to provide critical information to healthcare providers and patients, ensuring the safe and effective use of the medication.
Prescribing Information: CIPREMI (REMDESIVIR) for Injection and intravenous use (Sample)
Infographics
This infographic was created to convey complex medical information in a visually appealing and easy-to-understand format that helps in informed decision-making, encourages action, and builds trust.
Continuing Medical Education
This CME was created to help doctors, nurses, pharmacists, and other healthcare professionals stay updated with the latest medical knowledge, advancements in treatments, and best practices.
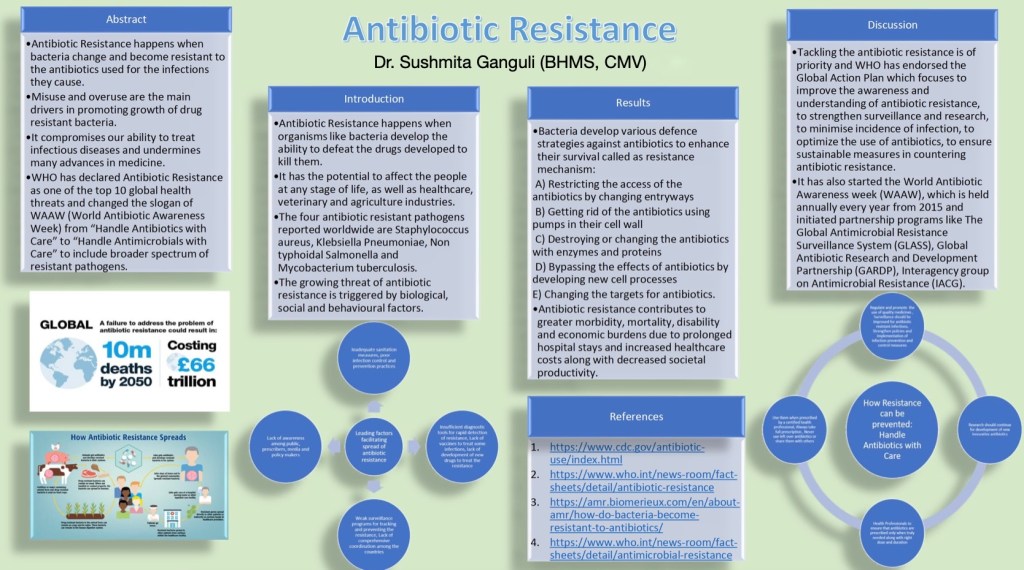
Scientific Poster
This submission-ready scientific poster is a highly effective tool for communicating scientific information to a wide audience in a concise and engaging format.
Health Journalism Article
This health journalism article aims to inform, educate, and empower the public on health-related issues by making complex medical information accessible and understandable.
Health Journalism Interview
This health journalism interview is conducted to gather expert insights while ensuring that the content is credible, informative, and relevant to the public.
Resume:
